Explain How the Characteristics Visible Light Generate Different Colors
A prism can separate the colors of white light dispersion because different frequencies of light have different refractive indices for a given material. Grass looks green because when light hits it the blades.
The colors of the visible spectrum.
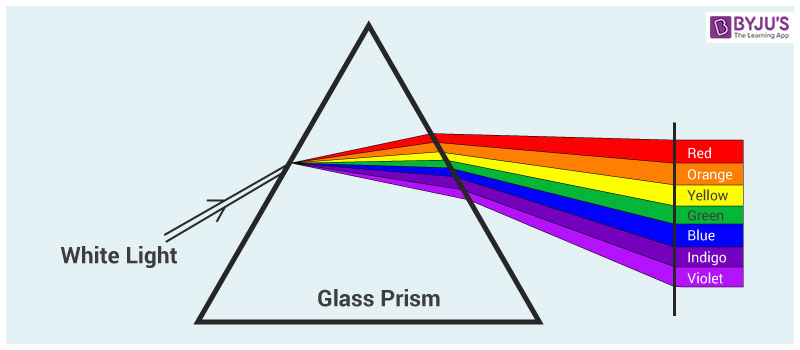
. Certain chemical of element reacts to lower temperature while the other reacts differently. As a result when white light bends through a prism each wavelength travels at a different speed inside the prism resulting in the different colors emerging from the. It has all the colors of the rainbow.
In the diagram below you can see the absorption spectra of three key pigments in photosynthesis. When we see an object of a certain color that means that light of that colors wavelength is being reflected off the object. The loosely held electrons of.
The colour of the light depends on the difference in energy between the two levels. Light is the relatively narrow frequency band of electromagnetic waves to which our eyes are sensitive. Fluorescent dyes absorb UV or blue light and use the energy absorbed to emit photons of different colors.
The colors of the sunset result from a phenomenon called scattering. Flame tests are a quick method of producing the characteristic colors of metallic ions we will talk more about ions later. The energy is supplied by the heat of the burning firework.
As light hits an object some light is absorbed and some is reflected. Yellow cyan and magenta. Identify and define the characteristics of electromagnetic radiation EMR used in microscopy.
When light hits an object some wavelengths are absorbed by that object and some are reflected. Cameras binoculars telescopes microscopes and the eye. Maxwells equations united the study of electromagnetism and optics.
Not only the element but the temperature of the heat is also different. When the electrons return to lower energy levels they emit energy in the form of light. Renowned researchers Thomas Young and Hermann von Helmholtz contributed to the trichromatic theory of color vision.
Wavelengths are usually measured in units of nanometers 1 nm 10 9 m or in units of angstroms 1Å 10 10 m. The theory began when Thomas Young proposed that color vision results from the actions of three different receptors. Heating an atom excites its electrons and they jump to higher energy levels.
Molecules and small particles in the atmosphere change the direction of light rays causing them to scatter. Light of different wavelengths looks like different colors to us. Visible light is part of the electromagnetic spectrum.
Figure illustrates the spectrum of visible light. This is often determined by the quality of the lenses and. In some cases the salts needed to produce the desired color are unstable.
As early as 1802 Young suggested that the eye contained different photoreceptor cells that were sensitive to different. Specific wavelengths within the spectrum correspond to a specific color based upon how. Any photographer who wishes to reach their full creative potential should likewise make it a point to understand the vital.
Light waves of different frequencies and wavelengths are distinguished as colors by the human eye. The color of the light depends on the specific energy change that is taking place. The heat excites the element and caused reaction that produce different color of light.
For example the red green and blue lines in the spectrum of hydrogen arise when the electron drops to level 2 from levels 3 4. Visible light - that which is detectable by the human eye - consists of wavelengths ranging from approximately 780 nanometer 780 x 10 -7 m down to 390 nanometer 390 x 10 -7 m. The energy of the photon determines its wavelength or color.
The set of wavelengths that a pigment doesnt absorb are reflected and the reflected light is what we see as color. This narrow band of frequencies is referred to as the visible light spectrum. When the electron returns to a lower energy state the energy is released in the form of a photon light.
Green-colored light lives between 540 and. The color of an object is the color of the light it reflects. All of the colors in the visible light spectrum are characterized by different frequencies.
Visible light is made up of different wavelengths and each color has its own unique wavelength. All other colors can be broken down into different combinations of. In order to harness the power dynamism and idiosyncratic nature of light it is absolutely necessary for a photographer to establish a real relationship with lightAs is true in human relationships understanding is a key component.
For example when you see a red shirt the shirt is absorbing all the colors of light except for the red. The resolving power is the optical instruments ability to produce separate images of two adjacent points. White light is a combination of all colors in the color spectrum.
Combining primary colors of light like red blue and green creates secondary colors. Diffraction of light plays a paramount role in limiting the resolving power of any optical instrument for example. Chlorophyll a chlorophyll b and β-carotene.
Electrons get to higher energy states. Therefore the temperature of the heat affects the color of the light.

Visible Light The Electromagnetic Spectrum Color

Color Theory Color Psychology Color Meanings

A Color Spectrum Chart With Frequencies And Wavelengths Science Struck Color Spectrum Visible Light Spectrum Visible Light

Comments
Post a Comment