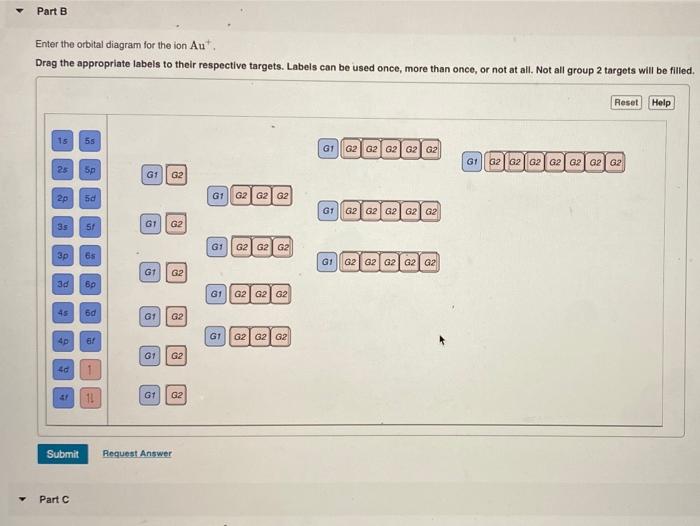
Enter the Orbital Diagram for the Ion Au+
Labels can be used once more than once or not at all. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d3 Mo is 42 on the Periodic Table since the question asks for Mo3 you have to subtract 3 electrons.
Solved 1 Enter The Orbital Diagram For The Ion Cd2 Drag Chegg Com
Electron Configuration Xe 4f14 5d10 6s1.

. Colored type indicates the sublevel to which the last electron is added. Part A The Lewis structure for the chlorate ion is O. Mo is element 42 so for the 3 ion you draw the configuration for.
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Draw an Molecular Orbital energy diagram and predict the bond order of L 2. Not all group 2 targets will be filled.
D0 ions d3 ions V2 Ta2 Cr3 Mo3 Mn4 etc. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy.
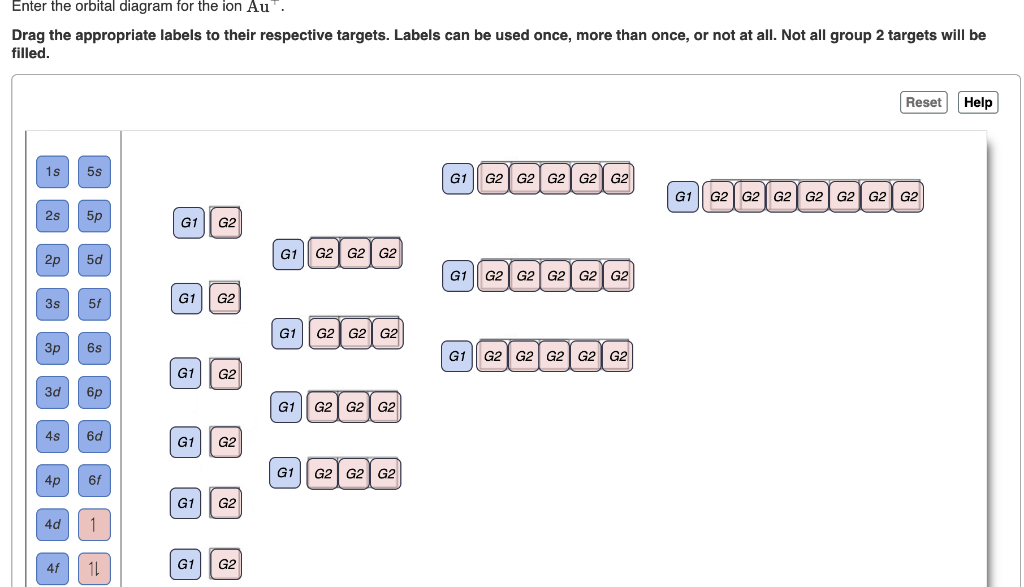
Electrons are generally removed from the s sub-level. Enter the orbital diagram for the ion Au. However even though the 5s orbital is lower in energy than the 4d orbital the electrons in the 4d orbitals shield the electron in the 5s orbitals.
Action here wants us to pick the electronic configuration for the following on. However even though the 5s orbital is lower in energy than the 4d orbital the electrons in the 4d orbitals shield the electron in the 5s orbitals. Click within the orbital to add electrons.
Using a partial orbital diagram show. Enter the orbital diagram for the ion Cd2. Construct the orbital diagram for the ion Mo3.
Add them in order of increasing orbital energy. For Au one electron is removed from the outermost 6s orbital making the configuration Xe4f 145d10. Enter the orbital diagram for the ion Au.
Enter the orbital diagram for the ion Au. Click within the orbital to add electrons. Orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the boxes with arrows s orbitalOrbital diagrams of atoms Diagram shows how the electrons are distributed among.
Part B Build the orbital diagram for the ion most likely formed by phosphorus. A the orbital speed increases as the planet approaches apogee B the planets orbital speed changes as it moves closer and farther from the sun C Chemistry Get Answer - 1. Use the buttons at the top of the tool to add orbitals.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 Mo 3. Write orbital diagrams for each ion and indicate whether the Quizlet. Drag the appropriate labels to their respective targets.
Calculate the formal charge on the chlorine Cl atom. The orbital diagram for gold starts with the base Xe which is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. The outer shells are 6s2 5d9.
Enter The Orbital Diagram For. Answer to Write orbital diagram for Au. Use the buttons at the top of the tool to add orbitals.
Figure Write orbital diagram for Au. Problem 78 Hard Difficulty. Add them in order of increasing orbital energy.
In a sense however were taking one orbital higher so one has to to as 22 p six three s 23 p six for. Enter The Orbital Diagram For The Ion Mo3. 1 Remove one electron from 5s1.
Enter the orbital diagram for the ion Cd2. 1s2 2s2 2p6 3s2 3p6 4s2 4p6 4d10. Enter the orbital diagram for the ion Au.
Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. Add them in order of increasing orbital energy. Thus the electron configuration of Cr3 is.
Express your answer as an integer. Use the buttons at the top of the tool to add orbitals. The atomic number of Au is Therefore its For Au one electron is removed from the outermost 6s orbital making the configuration.
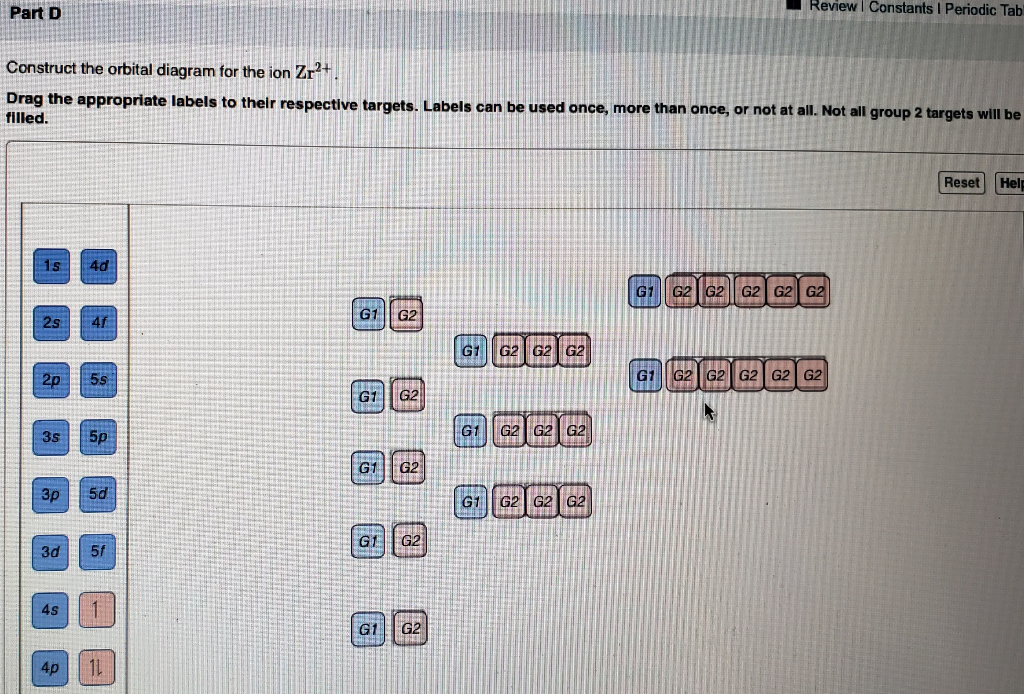
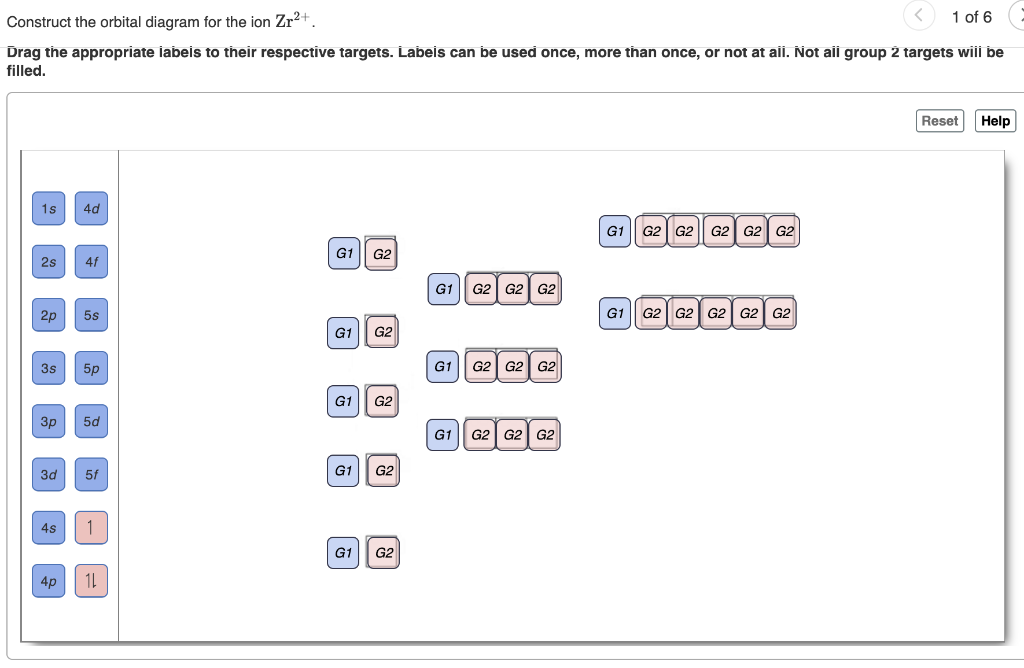
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. Therefore its configuration is. Enter the orbital diagram for the ion Au 3Construct the orbital diagram for the ion Mo3 4Construct the orbital diagram for the ion Zr2 Question.
σ-ML4 Tetrahedral MO Diagram e. When an element is a cation you REMOVE electrons. Click within the orbital to add electrons.
Labels can be used once more than once or not at all. 7 Express your answers. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10.
Enter the orbital diagram for the ion. The atomic number of Au is 79. Not all group 2 targets will be.
View Available Hints formal charge on CIEL Submit Part B Calculate the formal charge on each of the oxygen O atoms labeled a b and c in the following Lewis structure. Plus it would have the same configuration. Click within the orbital to add electrons.
So we have m g two plus um que plus c l minus and O to minus so energy to Plus is gonna have the conformation of one s two to s 22 p six and thatll be it for K. Can be accommodated in the metal d orbitals. Figure A periodic table of partial ground- state electron configurations.
However the chromium ion Cr3 possesses 24e 3e 21e due to the loss of 3 of its electrons. Figure Write orbital diagram for Au. Answer to Write orbital diagram for Mo3.
Jul 21 Write orbital diagram for Au Determine if the ion is diamagnetic or. Drag the appropriate labels to their respective targets. Labels can be used once more than once or not at all.
Enter the orbital diagram for the ion Cd2 Drag the appropriate labels to their respective targets.
Solved Enter The Orbital Diagram For The Ion Cd2 Drag The Chegg Com
Solved Part B Enter The Orbital Diagram For The Ion Au Drag Chegg Com
Solved Enter The Orbital Diagram For The Ion Cd2 Drag The Chegg Com
Comments
Post a Comment